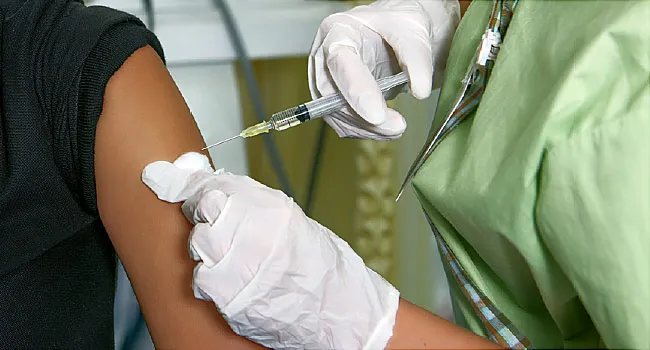
The FDA said it was basing its emergency authorization of boosters for 16 and 17 year olds on data from 200 individuals who were ages 18 to 55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in post-authorization studies.
from WebMD Health https://www.webmd.com/vaccines/covid-19-vaccine/news/20211209/fda-oks-boosters-for-teens?src=RSS_PUBLIC











0 Comments:
Post a Comment